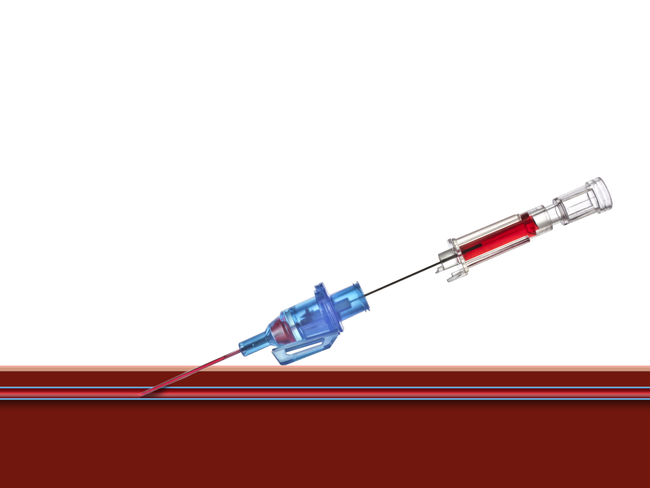
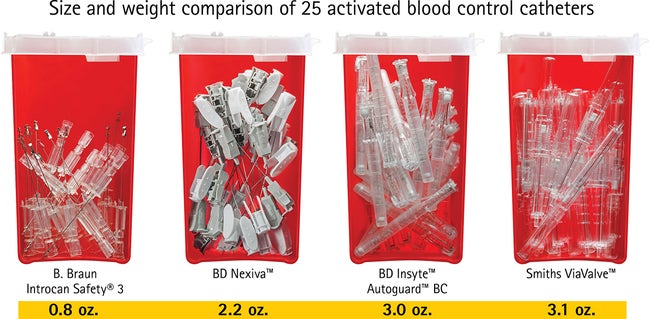
Introcan Safety® Gets the Job Done
IV access is an indispensable element of today’s infusion therapy. However, this invasive procedure continues to be associated with potential risks.1 At B. Braun, we developed the Introcan Safety® Family of IV Catheters with the guiding principle of making the job of IV access safe for the clinician.1 Introcan Safety gets the job done in every unit of the hospital, from the OR to the ICU to oncology.
Working together with clinical experts, B. Braun continues its proud tradition of excellence in safety with the Introcan Safety Family of IV Catheters. From insertion to catheter deployment to needle removal, clinicians are protected by a system that is activated automatically and cannot be bypassed. Learn more about how the B. Braun Introcan Safety Family can build confidence for clinicians and increase comfort for patients.
B. Braun is a proud sponsor of the Canadian Association of Critical Care Nurses (CACCN) and the Canadian Vascular Access Association (CVAA).
Introcan Safety Family of IV Catheters
Making Vascular Access Safe for Clinicians
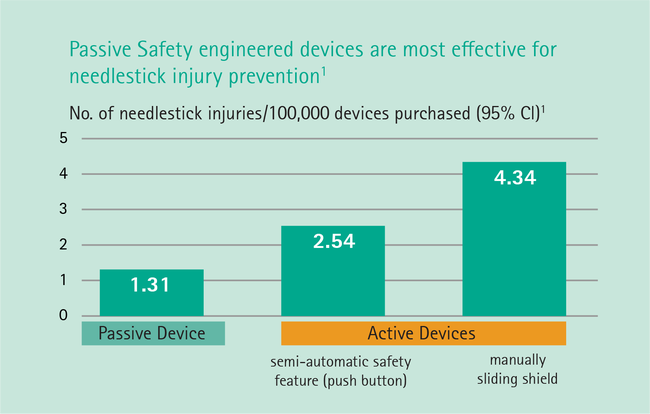
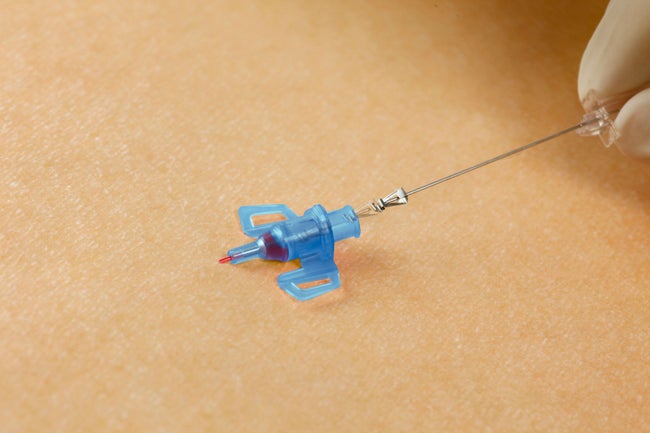
Needlestick injuries continue to be one of the highest risks clinicians will face during their daily routine. Studies show that passive, fully automatic safety devices are proven to be two times better than a semi-automatic ‘push-button’ safety shield and three times better than a manually sliding shield.1 With B. Braun’s family of Introcan Safety® IV catheters, clinicians are protected by a truly automatic passive safety device.
Our safety mechanism automatically engages upon needle removal, and can NOT be bypassed.
Making Vascular Access Efficient
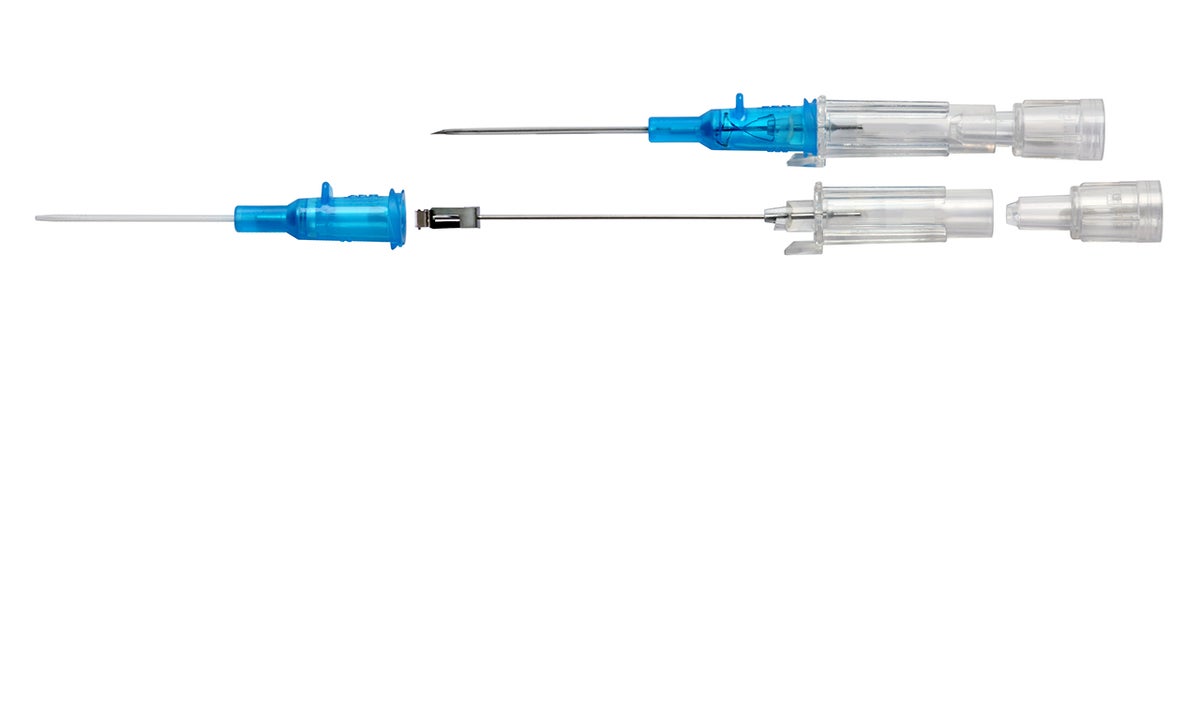
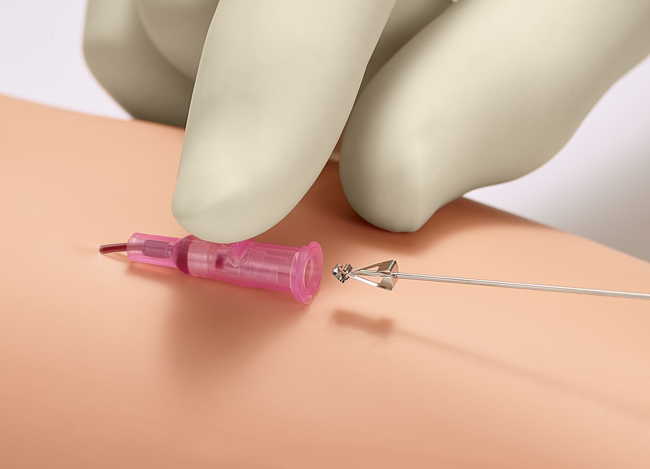
Multiple attempts to establish IV access can lead to a delay in treatment plus an increase in material cost and time. The Double Flashback Technology of the Introcan Safety® family of IV catheters supports first stick success through quick visual confirmation that the vessel has been punctured by both the needle and the catheter.
Making Vascular Access Convenient
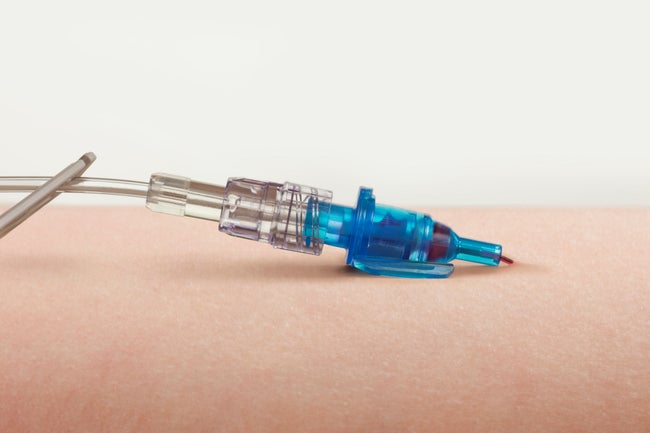
Nine out of 10 nurses reported that absence of blood leakage during short peripheral IV catheter insertion is important.2 The one-time Blood Control Septum on Introcan Safety 2 and the multi-access Blood Control Septum on Introcan Safety 3 are designed to restrict the flow of blood from the catheter hub after needle removal until first connection of a luer access device.
Survey findings show that the average cost per cleanup (when there's blood leakage from the catheter hub) is $0.30 for the gauze, absorbent drapes, towels, and sheets used per event.2 The cost may not seem expensive, but it adds up over time. The use of blood control IV catheters is designed to reduce cleanup time and materials.2
Economic and Environmental Savings in Vascular Access
Introcan Safety Family of IV Catheters
- Generate less waste with smaller, lighter components
- Save money and avoid throwing away unused components (compared to integrated catheters.)
- Cut costs by reducing needlesticks and cleanup time and materials
- PVC-Free, DEHP-Free and Latex-Free design protects the environment
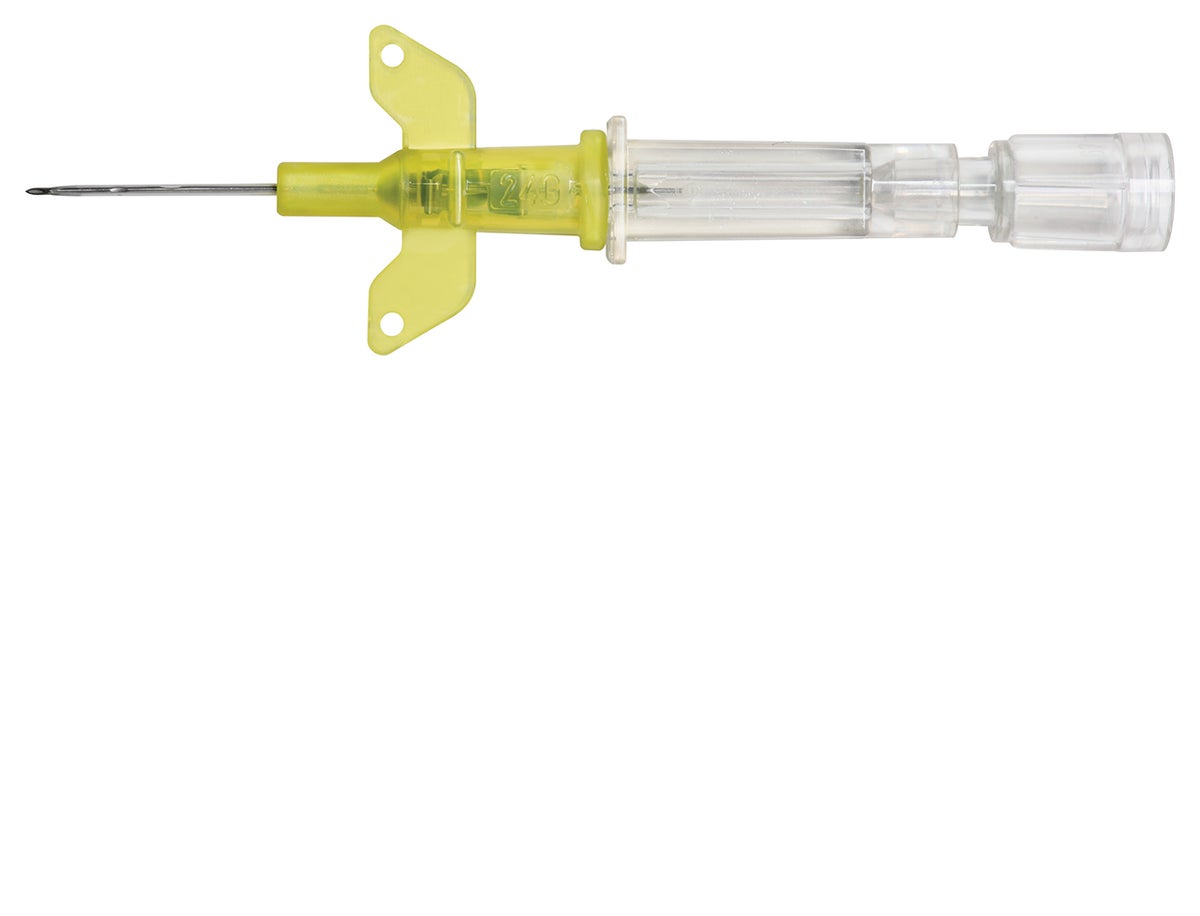
Introcan Safety IV Catheter
Although safety devices were introduced over a decade ago, preventing needlestick injuries continues to be a major concern. With the B. Braun Introcan Safety IV Catheter, you are protected by a truly passive safety device that is activated automatically and cannot be by-passed. No extra time-consuming steps are required to ensure healthcare worker and patient safety.
The Universal Bevel needle allows for a wider choice of insertion angles which makes insertions more comfortable for patients. With this easy-to-use safety IV catheter and its Double Flashback Technology, you can be confident of successful needle and catheter placement every time. And, with half the plastic content of other safety IV catheters, you can significantly reduce storage space and medical waste.
Introcan Safety 2 Multi-Access Blood Control
As the newest member of the family, Introcan Safety 2 is designed to automatically reduce the risk of blood exposure during insertion and every time the hub is accessed.1 Based on a clinical study, 9 out of 10 clinicians found Introcan Safety 2 IV Catheter easy to insert and 83% did not have to change their insertion technique.1
Introcan Safety 3 Closed IV Catheter
A fully automatic passive safety IV catheter with multiple access blood control and passive needlestick protection
As part of our continued effort to provide products that address the critical issues of infection prevention and healthcare worker and patient safety, B. Braun has developed the Introcan Safety 3 Closed IV Catheter, which automatically prevents needlesticks and blood exposure.
Its passive safety needle shield cannot be bypassed, aiding in the prevention of needlestick injuries. The multiple-access blood control septum aids in the prevention of blood exposure each time the catheter hub is accessed. Introcan Safety 3’s integrated stabilization platform improves catheter stability and minimizes movement within the vessel, reducing a number of catheter related complications. In addition, the advanced hub design allows for easier connections and less touch contamination while the catheter’s smooth ergonomic surface optimizes patient comfort.
Introcan Safety Deep Access IV Catheters
Approximately 1/3 of adults and up to 50% of children who require a PIVC have difficult venous access.4 Introcan Safety Deep Access provides clinicians with another choice when choosing the right line for their patients. Deep Access is designed to facilitate ultrasound-guided procedures and has shown a significant increase in catheter survival of 44 hours (1.8 days).4
Introcan Safety Benefits
Reducing Needlestick Injuries
The B. Braun Introcan Safety® Family of IV Catheters with Passive Safety Technology:
Deploy Automatically
- Safety feature requires no user activation — no button, twists or clicks
- Safety shield stays in place through disposal
Cannot Be Bypassed
- Unique, patented safety design automatically engages safety shield upon needle withdrawal
- Safety shield protects needle tip to prevent needlestick injury without any additional steps
Promote Compliance
- Encourages best practice by preventing needle reinsertion
Reduce Blood Exposure
B. Braun Introcan Safety 3’s multiple-access blood control septum:
Automatically controls blood exposure
- Reduce exposure to blood when the needle is removed and every time the hub is accessed
- Less stress for you and your patients
- Reduce clean up time and materials
Allows access to the hub multiple times
- For blood collection, boluses or set changes without having to worry about blood leakage
Promote First Stick Success
The B. Braun Introcan Safety® Family of IV Catheters help improve first stick success with:
Double Flashback Technology
- Helps ensure first stick success and patient comfort through quick visualization of both needle and catheter flashback
- Promotes best practice by reducing the need to remove and reinsert the needle in order to confirm catheter placement, as may occur with other "instant flash" systems
- Needle Flash confirms the needle is in the vessel
- Catheter Flash confirms catheter placement
Push-Off Plate
- Facilitates easy, one-hand catheter advancement
- Acts as a bevel orientation indicator
Locking Bevel Indicator
- Confirms correct bevel orientation (up)
- Adds stability during catheter advancement
Improve Patient Comfort
The B. Braun Introcan Safety® Family of IV Catheters enhance patient comfort with:
Universal Bevel Design
- Proprietary needle design to make catheter insertion more comfortable for patients
- Unique geometry provides a wide choice of insertion angles to aid in accessing difficult veins
- Super-sharp needle bevel helps improve patient comfort
- Creates a V-shaped, tricuspid incision versus a lancet cut for easier catheter insertion, less tissue tearing and faster healing
Optimized Catheter Lubrication
- Provides smooth, comfortable catheter advancement
Choice of Catheter Material
- Provides more comfortable indwelling performance options
- Polyurethane (PUR) for softer, more comfortable, longer indwelling and kink resistance (Introcan Safety® Introcan Safety® 2, Introcan Safety® 3 and Introcan Safety® Deep Access)
- Fluorinated ethylene propylene (FEP) with firmer construction for both arterial and venous access (Introcan Safety® only)
Ensure Best Practices
The B. Braun Introcan Safety® Family of IV Catheters is designed to be easy to use:
- No extra steps needed to prepare the catheter for insertion. It is ready to go right out of the package
- Safety shield deploys automatically and requires no activation — no buttons, no twists, no clicks to remember
- Push-Off Plate acts as a bevel orientation indicator and facilitates easy one-handed catheter advancement
- Locking Bevel Indicator confirms correct bevel orientation and adds stability during catheter advancement
- Removable flash plug permits attachment of a syringe for aspiration or other special procedures
- Promotes compliance by preventing needle reinsertion
Support Economic and Environmental Savings
The B. Braun Introcan Safety® Family of IV Catheters helps you cut costs and “go green.”
- Generate less waste with smaller, lighter components
- Save money and avoid throwing away unused components (compared to integrated catheters)
- Cut costs by reducing needlesticks and cleanup time and materials
- Not made with PVC, DEHP, or natural rubber
- Tosini W, Ciotti C, Goyer F, Lolom I, L’Heriteau F, Abiteboul D, et al. Needlestick Injury Rates According to Different Types of Safety-Engineered Devices: Results of a French Multicenter Study. Infect Control Hosp Epidemiol. 2010 Apr; 31(4):402-7
- Richardson D, “Reducing blood exposure risks and costs associated with SPIVC insertion”, Nursing Management, vol. 42, no. 12, Dec 2011 (reprint p. 1-4).
- Data on File.
- Whalen, M., Maliszewski, B. and Baptiste, D.L., 2017. Establishing a dedicated difficult vascular access team in the emergency department: a needs assessment. Journal of Infusion Nursing, 40(3), pp.149-154.
- Amit Bahl, MD, MPH; Mahmoud Hijazi, BA; Nai-Wei Chen, PhD; Ludovic Lachapelle-Clavette, BA; Jacob Price, MD. (2020). Ultralong Versus Standard Long Peripheral Intravenous Catheters: A Randomized Controlled Trial of Ultrasonographically Guided Catheter Survival. Ann Emerg Med. pii S0196-0644(19)31383-6. doi: 10.1016/j.annemergemed.2019.11.013.
- Data on File.