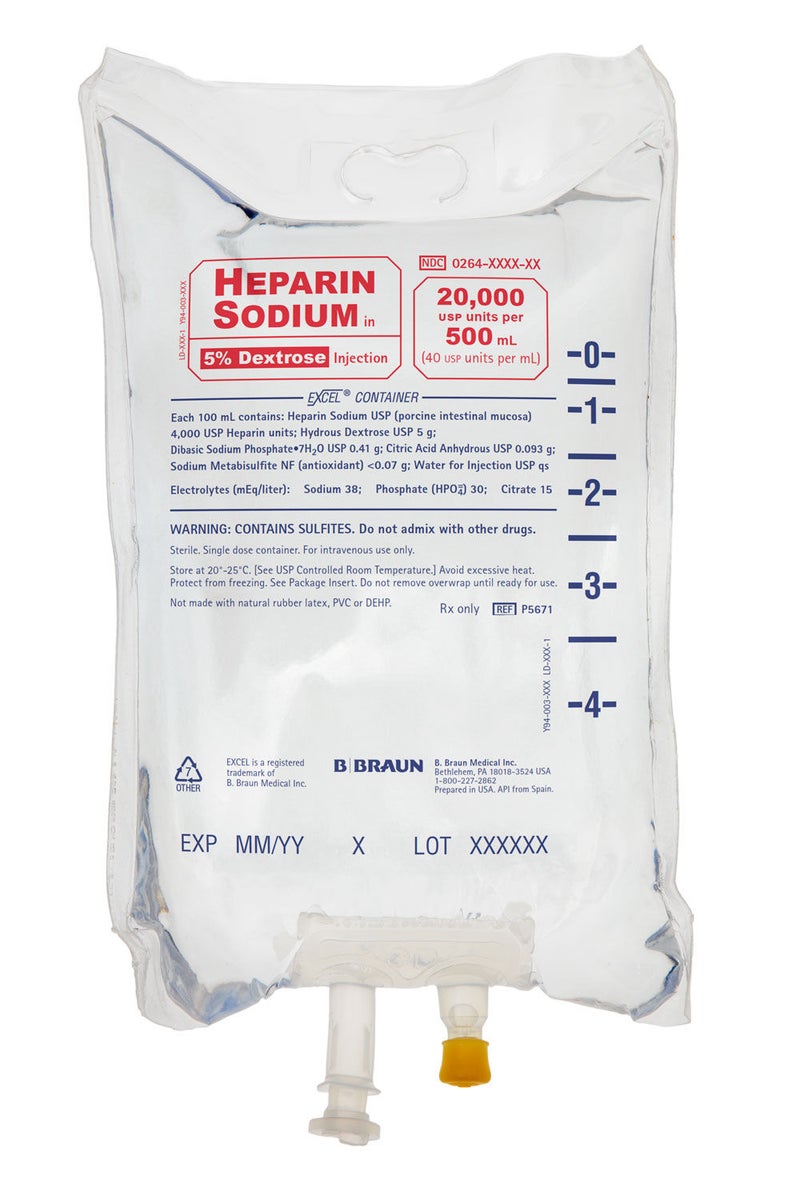
20,000 Units Heparin in 5% Dextrose Injection, 40 Units mL, Canada Only
500 mL EXCEL® IV Container
Heparin Sodium in 5% Dextrose Injection is a sterile, nonpyrogenic solution prepared from heparin sodium (derived from porcine intestinal mucosa and standardized for use as an anticoagulant) and Hydrous Dextrose USP in Water for Injection USP. It is to be administered by intravenous injection. The potency is determined by a biological assay using a USP reference standard based on units of heparin activity per milligram.
Not made with natural rubber latex, PVC or DEHP.
DIN 02209713
Specifications
| Product Code | P5671-00 |
|---|---|
| NDC Number | 00264-9567-24 |
| Reference Number | P5671-00 |
| DEHP Information | Components Do Not Contain DEHP |
|---|---|
| Latex Information | Components Do Not Contain Natural Rubber |
| PVC Information | Components Do Not Contain PVC |
| Total Shelf Life (Months) | 018 |
|---|