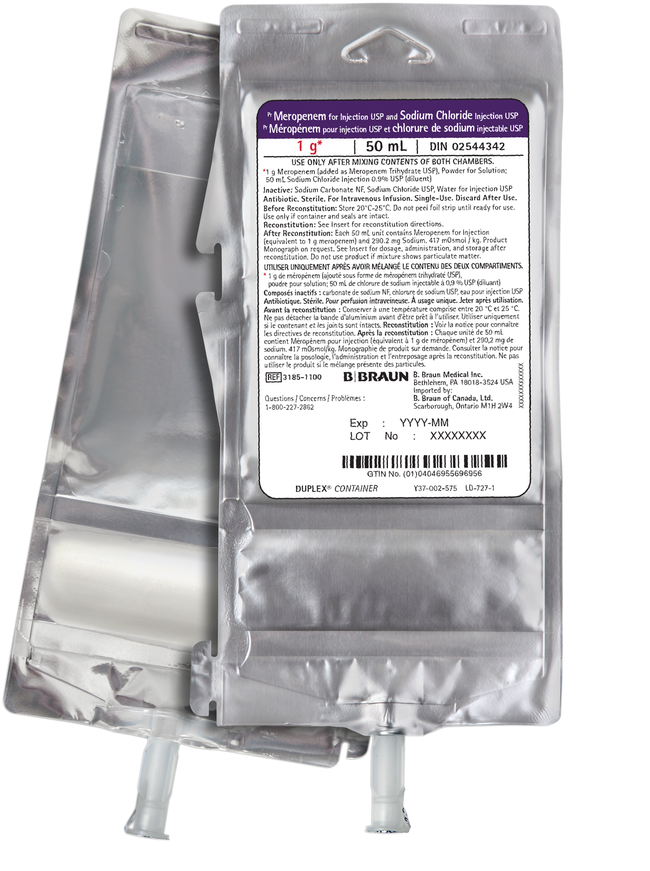
Meropenem for Injection USP and Sodium Chloride Injection USP in DUPLEX® Container
Available in Ready-to-Use Duplex container in doses of 500 mg/50 mL and 1 g/50 mL.
Meropenem is a broad spectrum, β-lactamase-resistant, carbapenem antibiotic for parenteral administration.1
| DIN | Product Code | Container | Case Quantity |
| 02544334 | 3183-1100 | 500mg DUPLEX | 24 |
| 02544342 | 3185-1100 | 1g DUPLEX | 24 |
Ready When You Are in 5 Steps!
Directions for Use
Meropenem for Injection USP and Sodium Chloride Injection USP is packaged in a sterile, nonpyrogenic, single-dose Duplex Container with meropenem in the drug chamber and 50 mL of 0.9% sodium chloride solution in the diluent chamber.
- The first Meropenem for Injection in a ready-to-use format in Canada
- Duplex containers do not need an overwrap and are designed to reduce waste
- The Duplex container is not made with natural rubber latex, PVC, or DEHP
- Closed System container designed to prevent contamination and maintain potency
The Duplex container is designed to reduce the potential risks associated with drug delivery.
- Two compartment container ensures diluent cannot be delivered without the drug.
- Ready-to-use container keeps pre-measured medication and diluent separate until you’re ready to mix and administer.
- Helps reduce medication errors.
- No need for multiple admixture SKUs which can result in savings of time, resources and labour.
- Designed to be economical and reduce drug waste.
- Stored at room temperature for up to 36 months.1
- Conveniently fits in automated dispensing cabinets.
Additional Information1
INDICATIONS AND USAGE
Meropenem is indicated for treatment of the following infections when caused by susceptible strains of the designated micro-organisms:
- Lower Respiratory Tract
- Urinary Tract
- Intra-abdominal
- Gynecologic
- Uncomplicated Skin and Skin Structure
- Complicated Skin and Skin Structure
- Bacterial Meningitis
- Bacterial Septicemia
CONTRAINDICATIONS
- Hypersensitivity to meropenem, to any ingredient in the formulation, including any non-medicinal ingredient, or component of the container
- In patients who have demonstrated anaphylactic reactions to β-lactam antibiotics
- Where the administration of sodium chloride could be clinically detrimental
Related Documents
References
1. Product Monograph B. Braun Meropenem Injection January 2024